Unit 6 – Solutions (Short Questions)
Q.1 Why suspensions and solutions do not show Tyndall effect, while colloids do?
Q.2 What is the reason for the difference between solutions, colloids and suspensions?
Q.3 Why does not the suspension form a homogeneous mixture?
Q.4 How will you test whether given solution is a colloidal solution or not?
Q.5 Classify the following into true solution and colloidal solution Blood, starch solution, glucose solution, tooth paste, copper sulphate solution, silver nitrate solution.
Q.6 Why we stir paints thoroughly before using?
Q.7 Which of the following will scatter light and why? Sugar solution, soap solution and milk of magnesia.
Q.8 What do you mean by “like dissolves like?” Explain with examples.
Q.9 How does nature of attractive forces of solute-solute and solvent-solvent affect the solubility?
Q.10 How you can explain the solute-solvent interaction to prepare a NaCl solution?
Q.11 Justify with an example that solubility of a salt increases with the increase in temperature
Q.12 What do you mean by volume/volume %?
Q.1 Why suspensions and solutions do not show Tyndall effect, while colloids do?
Answer:
Suspensions and solutions do not show Tyndall effect because in suspensions particles are so big that light is blocked and difficult to pass. But in solution particles are so small that they cannot scatter the rays of light, thus do not show Tyndall effect. But colloids can show Tyndall effect because particles scatter the path of light rays thus emitting the beam of light i.e., exhibit the Tyndall effect.
Q.2 What is the reason for the difference between solutions, colloids and suspensions?
Answer:
The differentiation between solutions, colloids and suspensions is based upon the particle size. In colloidal solutions the particles size is intermediate between true solutions and suspensions.
Q.3 Why does not the suspension form a homogeneous mixture?
Answer:
In suspension particles remain un-dissolved due to their big size. After sometime particles settle down under the action of gravity, therefore suspension does not forma homogeneous mixture.
Q.4 How will you test whether given solution is a colloidal solution or not?
Answer:
We will pass light in the solution, if the given solution scattered the light then it is a colloidal solution. It solution does not scatter the light then it is not colloidal solution.
Q.5 Classify the following into true solution and colloidal solution Blood, starch solution, glucose solution, tooth paste, copper sulphate solution, silver nitrate solution.
Answer:

Q.6 Why we stir paints thoroughly before using?
Answer:
Paints are heterogeneous mixture of un-dissolved particles in a given medium. Particles settle down after sometime. So we stir paints to mix thoroughly before using.
Q.7 Which of the following will scatter light and why? Sugar solution, soap solution and milk of magnesia.
Answer:
Soap solution:
Soap solution will scatter light (Tyndall effect) because it is colloidal solution and its particles are large enough to scatter the light.
Sugar Solution:
Sugar solution will not scatter light because the particles of sugar solution are so small that they cannot scatter light. Milk of Magnesia:
Milk of magnesia cannot scatter the light because it is suspension and its particles are so big that light is blocked.
Q.8 What do you mean by “like dissolves like?” Explain with examples.
Answer:
“Like dissolves like” means that polar substances are dissolved in polar solvents and non polar substances are soluble in non polar solvents.
For example: NaCl (polar) dissolves in water (polar solvent) and does not dissolve in benzene (non polar)
Q.9 How does nature of attractive forces of solute-solute and solvent-solvent affect the solubility?
Answer:
Solubility depends upon solute solvent attractions. If the attractive forces between solvent are stronger the solubility is greater. If the attractive forces become weaker in solute there will be greater solubility.
If the attractive forces between solute particles are stronger than solute solvent forces, solute remains insoluble and solution is not formed.
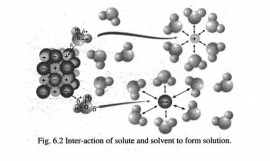
Q.10 How you can explain the solute-solvent interaction to prepare a NaCl solution?
Answer:
When NaCl is added in water it dissolves readily because the attractive forces between the ions of NaCl and polar molecules of water are strong enough to overcome the attractive forces between Na+ and Cl- ions in solid NaCl crystal. In this process, positive end of the water dipole is oriented towards the Cl ions and the negative end of water dipole is oriented towards the Nations. These ion-dipole attractions between Na+ ions and water molecules, Cl’ ions and water molecules are so strong that they pull these ions from their positions in the crystal and thus NaCl dissolves.
Q.11 Justify with an example that solubility of a salt increases with the increase in temperature
Answer:
Solubility of some salts which are usually ionic in nature increases with the increase in
temperature for such solutes. It means that heat is required to break the attractive forces between the ions of solute. This process is called endothermic.
For example:
Solubility of KNO3 and KCl can be enhanced by increasing temperature.
Q.12 What do you mean by volume/volume %?
Answer:
