Unit 3 – Periodic Table and Periodicity of Properties (Long Questions)
Q.1 Explain the contributions of Mendeleev for the arrangement of elements in a Periodic Table.
Q.2 Show why in a ‘period’ the size of an atom decreases if one moves from left to right.
Q.3 Describe the trends of electronegativity in a period and in a group.
Q.4 Discuss the important features of modem Periodic Table.
Q.5 What do you mean by blocks in a periodic table and why elements were placed in blocks?
Q.6 Discuss in detail the periods in Periodic Table?
Q.7 Why and how elements are arranged in a Periodic Table?
Q.8 What is ionization energy? Describe its trend in the Periodic Table?
Q.9 Define electron affinity, why it increases in a period and decreases in a group in the Periodic Table.
Q.10 Justify the statement, bigger size atoms have low ionization energy and have more shielding effect.
Q.1 Explain the contributions of Mendeleev for the arrangement of elements in a Periodic Table.
Answer:
Mendeleev’s Periodic Table
Introduction:
A Russian chemist, Mendeleev arranged the known elements (only 63) in order of increasing atomic masses, in horizontal rows called periods, so that elements with similar properties were in the same vertical columns. This arrangement of elements was called Periodic Table.
Mendeleev’s Periodic law,
“Properties of the elements are periodic functions of their atomic masses” Demerits of Mendeleev’s periodic table:
i. It did not explain the position of isotopes.
ii. Wrong order of the atomic masses of some elements suggested that the atomic mass of an element cannot serve as the basis for the arrangement of elements.
Q.2 Show why in a ‘period’ the size of an atom decreases if one moves from left to right.
Answer:
Atomic size or atomic radius:
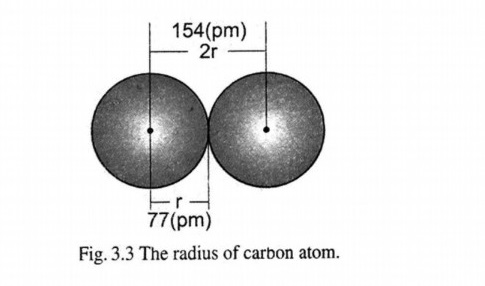
“The half of the distance between the nuclei of the two bonded atoms is referred to as the atomic radius of the atom.
Example:
The distance between the nuclei of two carbon atoms in its elemental form is 154 pm, means its half 77 pm is radius of carbon atom.
Q.3 Describe the trends of electronegativity in a period and in a group.
Answer:
Electronegativity:
“The ability of an atom to attract the shared pair of electrons towards itself in a molecule is called electronegativity.”
Explanation:
It is an important property especially when the covalent type of bonding of elements is under consideration.
Trends in periods:
Electronegativity increases from left to right in the periodic table. The trend of electronegativity is the same as ionization energy and electron affinity. It increases in a period from left to right.
Reason:
Because higher (Zeff) shortens the distance from the nucleus of the shared pair of electrons. Thus, enhances the power to attract the shared pair of electrons.
Example:
Electronegativity values of group 17 are given as follows:
Trend in groups:
Electronegativity decreases from top to bottom in the group. It generally decreases down a group because of the size of the atom increases. Thus attraction for the shared pair of electrons weakens. For example, electronegativity values of group 17 (halogens) are presented here.
17th group
Q.4 Discuss the important features of modem Periodic Table.
Answer:
The salient features of the long form of the periodic table are as follows:
Periods:
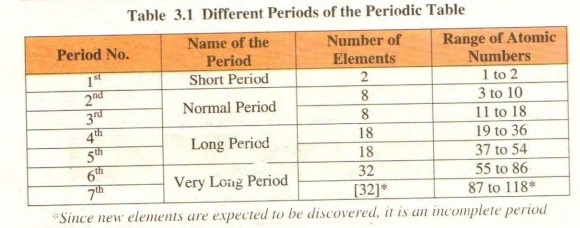
i. This table consists of seven horizontal rows called periods
ii. First period consists of only two elements. Second and third period consist of 8 elements each. Fourth and fifth period consist of 18 elements each. Sixth period has 32 elements while seventh period has 32 elements and is incomplete.
iii. Elements of a period show different properties.
Groups:
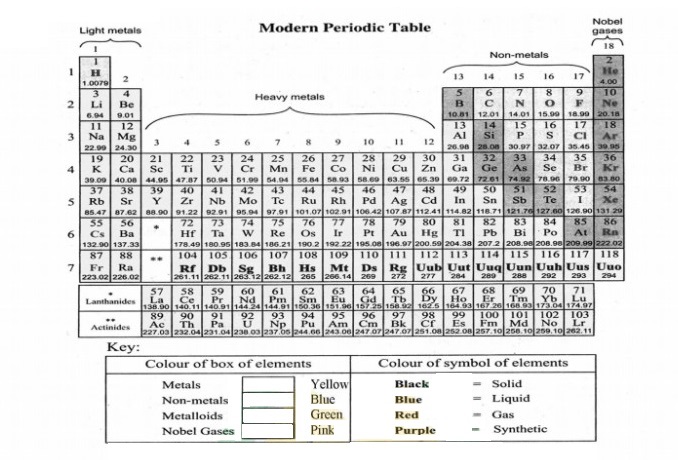
i. There are 18 vertical columns in the periodic table numbered 1 to 18 from left to right, which is called groups.
ii. The elements of a group show similar properties.
Blocks:
i. Elements are classified into four blocks depending upon the type of the sub-shell which gets the last electron.
Q.5 What do you mean by blocks in a periodic table and why elements were placed in blocks?
Answer:
Blocks of elements:
On the basis of completion of a particular sub-shell, elements with similar sub-shell the electronic configuration is referred to as a block of elements.
Types of blocks:
i. There are four blocks in the periodic table named after the name of the sub-shell which is in the process of completion by the electrons.
įi. These are s, p, d, and f blocks in the periodic table.
a. s-block:
The elements in which valence electrons are present in the s-subshell are called s-block elements.” “Elements of groups 1 and 2 have valence electrons in ‘s’ subshell. Therefore, they are called s-block elements.”b. p-block:
The elements in which valence electrons are present in the p-subshell are called p-block elements.”
“Elements of group 13 to 18 have their valence electrons in ‘p’ subshell. Therefore, they are referred to as p-block elements.”
c. d-block:
The elements in which valence electrons are present in the d-subshell are called d-block elements.”
“The elements of group 3 to group 12 have their valence electrons in d subshell.
Therefore they are called d-block elements. The d-block constitutes periods 4, 5, and 6. Each period in the d-block consists of ten groups starting from group 3 to group 12. These are called transition metals.”
d. f-block:
The elements in which valence electrons are present in the f-subshell are called f-block elements.” “f-block lies separately at the bottom of the periodic table. It consists of lanthanides and actinides.
Q.6 Discuss in detail the periods in Periodic Table?
Answer:
Periods:
“Horizontal rows of elements in the periodic table are called periods.” There are seven periods in the modern periodic table. The period number of an element represents the number of shells in the element.
First period:
It is called a short period. It consists of only two elements, hydrogen, and helium.
Second and third periods:
These are called normal periods. Each of them has eight elements in it. The second period consists of lithium beryllium, boron, carbon, nitrogen, oxygen, fluorine, and ends at neon, a noblegas.
Fourth and fifth periods:
These are called long periods. Each one of them consists of eighteen elements. Sixth and seventh periods: These are called very long periods. The sixth period contains 32 elements whereas the seventh period is incomplete.
Lanthnides and actinides:
In sixth and seventh periods after atomic numbers 57 and 89, two series of fourteen elements each were accommodated.
a. Why lanthanides and actinides are placed separately?
Because of the space problems, these two series were placed separately below the normal periodic table to keep it in a manageable and presentable form.
b. Why lanthanides and actinides are called so?
Since the two series start after Lanthanum (Z=57) and Actinium (Z=89), so these two series of elements are named Lanthanides and Actinides respectively.
Starting and ending of a period:
All the periods, except the first period, start with an alkali metal and end at a noble gas. It is to be observed that the number of elements in a period is fixed because of the maximum number of electrons that can be accommodated in the particular valence shell of the elements.
Q.7 Why and how elements are arranged in a Periodic Table?
Answer:
Modern Periodic Table:
“A table obtained by the arrangement of elements into groups and periods in increasing order of their atomic number is called the modern periodic table.” Atomic number of an element is a more fundamental property than atomic mass
i. It increases regularly from element to element.
ii. It is fixed for every element. So the discovery of atomic number of an element in 1913 led to a change in Mendeleev’s periodic law which was based on atomic mass.
Basis of Modern Periodic Table:
The modern periodic table is based upon the arrangement of elements according to increasing atomic numbers. When the elements are arranged according to increasing atomic number from left to right in a horizontal row, properties of elements were found repeating after regular intervals such that elements of similar properties and similar configuration are placed in the same group. It was observed that after every eighth element, the ninth element had similar properties to the first element.
Example:
Sodium (Z=11) had similar properties to lithium (Z=3). After atomic number 18, every nineteenth element was showing similar behaviour. So the long rows of elements were cut into rows of eight and eighteen elements, and placed one above the other so that a table of vertical and horizontal rows was obtained.
Q.8 What is ionization energy? Describe its trend in the Periodic Table?
Answer:
Ionization Energy (I.E)
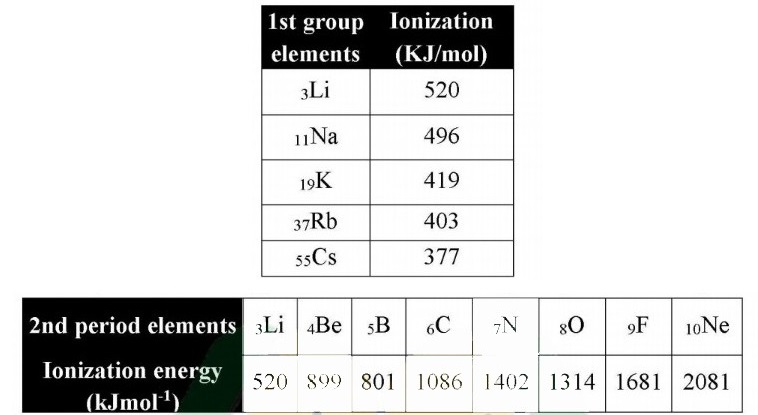
“The amount of energy required to remove the most loosely bound electron from the valence shell of an isolated gaseous atom is called ionization energy.” Units of I.E: The units of ionization energy are KJ mol-‘ and ev/atom. First Ionization Energy: The amount of energy required to remove the first electron from the valence shell of an isolated gaseous atom is called first ionization energy.
Example:
The first ionization energy of the sodium atom is + 496 kJmol-1
![]()
Second ionization energy:
“The amount of energy required to remove the second electron from the valence shell of an isolated gaseous mono positive ion is called second ionization energy.” When there is more than one electron in the valence shell they can be removed one by one providing more and more energy. Such as group 2 and 3 elements have more than one electron in their valence shells. Therefore, they will have more than one ionization energy value For example:

Third ionization energy: “The amount of energy required to remove the third electron from the valence shell of
an isolated gaseous di-positive ion is called third ionization energy.”
Trends along with groups
Ionization energy of elements decreases from top to bottom in a group. Reason:
i. The number of shells increases
ii. The distance between the nucleus and valence shells increases.
iii. Shelling effect increases.
iv. Nuclear attraction on valence electrons decreases.
v. Nuclear attraction on valence electrons increases.
Therefore, ionization energy increases from left to right in periods of the periodic table.
Q.9 Define electron affinity, why it increases in a period and decreases in a group in the Periodic Table.
Answer:
Electron Affinity
“The amount of energy released when an electron is added up in the outermost shell of an isolated gaseous atom is called electron affinity.” Example:
The electron affinity of fluorine is -328 kJ mol-1 i.e. one mole atom of fluorine releases 328 kJ of energy to form one mole of fluoride ions.
F+le — >F
AH = -328 KJ mol-1 Affinity means attraction. Therefore, electron affinity means tendency of an atom to
accept an electron to form an anion. Units of electron affinity:
The units of electron affinity are KJmol and eV/atom. Trend of electron affinity along period:
Electron affinity values increase from left to right in the period. . Reason:
The reason for this increase is, as the size of atoms decreases in a period, the attraction of the nucleus for the incoming electron increases. That means more is
attraction for the electron, more energy will be released. Trend of electron affinity along group:
In a group electron affinity values decrease from top to bottom because the size of elements of atoms increases down the group.
Reason:
With the increase in the size of atom shielding effect increases that results in poor attraction for the incoming electron i.e. less energy is released out. For example, as the size of an iodine atom is bigger than chlorine, its electron affinity is less than chlorine.

Q.10 Justify the statement, bigger size atoms have low ionization energy and have more shielding effect.
Answer:
Ionization Energy:
The amount of energy required to remove the most loosely bound electron from the valence shell of an isolated gaseous atom is called ionization energy.”
Shielding Effect:
“The decrease in the attractive force exerted by the nucleus on the valence shell electrons due to the presence of the electrons lying between the nucleus and valence shell is called shielding effect.”
As we move down the group more and more shells lie between the valence shell and the nucleus of the atom, these additional shells reduce the electrostatic force felt by the electron present in the outermost shell which results in more shielding effect by such bigger size atoms. Resultantly the valence shell electrons can be released easily. Therefore bigger size atoms have more shielding effect and low ionization energies.