Unit 1 – Fundamentals of Chemistry (Long Questions)
Q.1. Define elements and classify the elements with example.
Q.2. List five characteristics by which compounds can be distinguished from mixtures.
Q.3. Differentiate between the following with examples:(a) Molecule and gram molecule (b) Atom and gram atom(c) Molecular mass and molar mass (d) Chemical formula and gram formula
Q.4 Mole is SI unit for the amount of a substance. Define it with examples?
Q.1. Define elements and classify the elements with example.
Answer:
-
Elements
Historical Background:
Number of elements in early ages: In the early ages, only nine elements (carbon, gold, silver, tin, mercury, lead, copper, iron and sulphur) were known.
Previous Definition:
In early ages the element was defined as:
“The substances that could not be broken down into simpler units by ordinary chemical processes are called elements.” -
Number of elements until the end of 19th century: Until the end of nineteenth century sixty-three elements had been discovered.
Present number of elements: - Now 118 elements have been discovered, out of which 92 are naturally occurring
elements.
Modern definition “A substance made up of same type of atoms, having same atomic number and it cannot be decomposed into simple substances by ordinary chemical means is called an element.”
Occurrence of elements
Elements occur in nature in free or combined form. All the naturally occurring elements in the world have different percentages in the earth’s crust, oceans and atmosphere.
Table 1.1 Natural Occurrences by weight % of some major elements
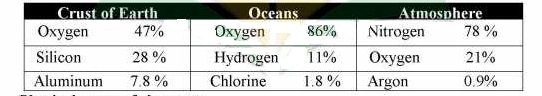
Physical states of elements:
Elements may be solids, liquids or gases.
i. Solids:
Majority of the elements exist as solids e.g. Sodium, copper, zinc, gold
ii. Liquid:
There are very few elements which occur in liquid state e.g. Mercury and bromine
iii. Gases:
A few elements exist as gases e.g
Nitrogen, oxygen, chlorine and hydrogen
Classification of elements:
On the basis of their properties, elements are divided into:
Metals: e.g. sodium, copper. Zinc, gold etc About 80 percent of the elements are metals.
Non-metals: e.g.: nitrogen oxygen, carbon etc. Metalloids: Elements which have proportion of both metals and non metals are called metalloids or semi metals e.g. germanium, arsenic, antimony etc.
Q.2. List five characteristics by which compounds can be distinguished from mixtures.
Answer:
Difference between a compound and a mixture:
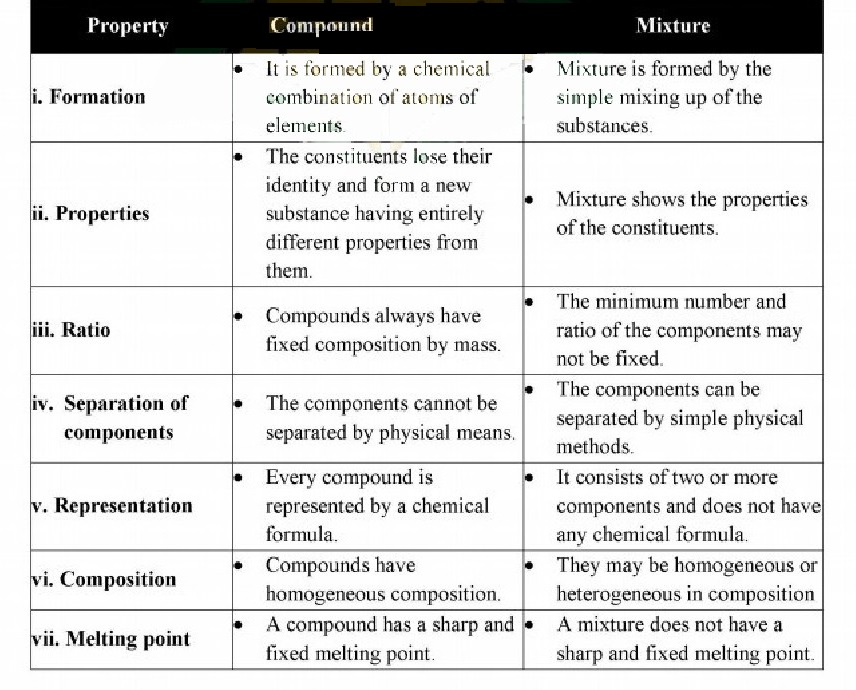
Q.3. Differentiate between the following with examples:
(a) Molecule and gram molecule (b) Atom and gram atom
(c) Molecular mass and molar mass (d) Chemical formula and gram formula
Answer:
Gram Atomic Mass (Gram atom or mole)
“The atomic mass of an element expressed in grams is called gram atomic mass or gram atom. It is also called a mole”.
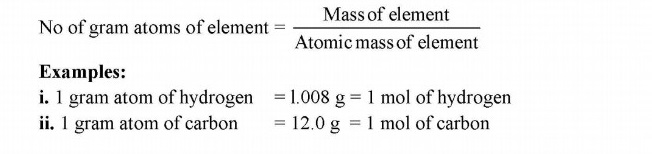
It means that 1 gram atom of different elements has different masses.
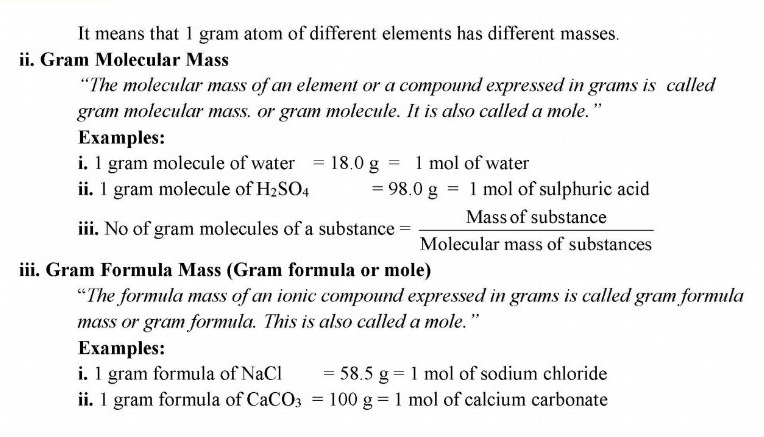
Q.4 Mole is SI unit for the amount of a substance. Define it with examples?
Answer:
Mole (chemist secret unit) Quantitative definition:
“A mole is defined as the amount (mass) of a substance that contains 6.02 x 1023 number of particles (atoms, molecules or formula units) is called a mole.”
Qualitative definition:
“The atomic mass molecular mass formula mass or ionic mass of a substance expressed in grams is called a mole.”
Symbol:
It is abbreviated as “mol” when it is used as a unit.
Explanation:
Mass of a substance is either one of the following: atomic mass, molecular mass or formula mass. These masses are expressed in atomic mass units (amu). But when these masses are expressed in grams, they are called as molar masses or molar mass of a substance.
Quantitative definition of mole
It is the atomic mass, molecular mass or formula mass of a substance expressed in grams is called mole.
Examples:
i. Atomic mass of carbon expressed in gram = 12g = 1 mole of carbon
ii. Molecular mass of H2O expressed in gram =18g = I mole of water
iii. Molecular mass of H2SO4 expressed in gram = 98 g = 1 mole of H2SO4
iv. Formula mass of NaCl expressed in gram = 58.5g = 1 mole of NaCl Relationship between mole and mass:
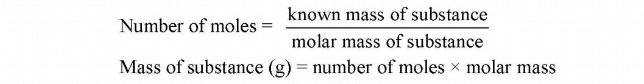
Relationship between mole and number of particles:
Number of particles = number of moles x 6.02×1023