Unit 4 – Structure of Molecules (Short Questions)
Q.1 Why do atoms react?
Q.2 Why is the bond between an electropositive and an electronegative atom ionic in nature?
Q.3 Ionic compounds are solids. Justify.
Q.4 More electronegative elements can form bonds between themselves. Justify.
Q.5 Metals are good conductor of electricity. Why?
Q.6 Ionic compounds conduct electricity in solution or molten form. Why?
Q.7 What type of covalent bond is formed in nitrogen molecule?
Q.8 Differentiate between lone pair and bond pair of electron.
Q.9 Describe at least two necessary conditions for the formation of a covalent bond.
Q.10 Why HCl has dipole-dipole forces of attraction?
Q.11 What is a triple covalent bond, explain with an example?
Q.12 What is difference between polar and non-polar covalent bonds, explain with one example of each?
Q.13 Why a covalent bond becomes polar?
Q.14 What is relationship between electronegativity and polarity?
Q.15 Why does ice float on water?
Q.16 Give the characteristic properties of ionic compounds.
Q.17 What characteristic properties do the covalent compounds have?
Q.1 Why do atoms react?
Answer:
Atoms react to form chemical bonds in order to get stability. Atoms achieve stability by attaining electronic configuration of inert gases by losing, gaining or sharing of electron.
Q.2 Why is the bond between an electropositive and an electronegative atom ionic in nature?
Answer:
The bond between an electropositive and an electronegative atom is ionic in nature because electropositive atom due to low I.E. can lose electron easily and forms a positive ion whereas electronegative atom due to high electron affinity will accept that electron easily and forms a negative ion. In this way positive and negative ions are attracted by electrostatic force of attraction to form ionic bond.
Q.3 Ionic compounds are solids. Justify.
Answer:
Ionic compounds are solids because they have strong electrostatic forces of attraction between positively and negatively charged ions which hold them in a three dimensional crystalline or solid form. Example: Potassium chloride (KCl) is a crystalline solid.
Q.4 More electronegative elements can form bonds between themselves. Justify.
Answer:
More electronegative elements have high values of ionization energy and do not lose electrons. They share electrons between their own atoms to complete their valence shells and form covalent bond.
Q.5 Metals are good conductor of electricity. Why?
Answer:
Metals are good conductors of electricity due to presence of mobile or free electrons.
Q.6 Ionic compounds conduct electricity in solution or molten form. Why?
Answer:
Ionic compounds conduct electricity in solution or molten form because in these two states ionic compounds have free ions in them. When these free ions move in solution or molten state they become conductor of electricity.
Q.7 What type of covalent bond is formed in nitrogen molecule?
Answer:
Triple covalent bond is formed in nitrogen molecule. In nitrogen molecule three bond pairs are involved in bond formation.
![]()
Q.8 Differentiate between lone pair and bond pair of electron.
Answer:
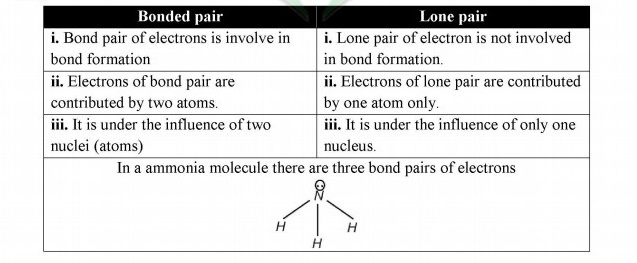
Q.9 Describe at least two necessary conditions for the formation of a covalent bond.
Answer:
Necessary conditions:
a. Elements should be electronegative in nature.
b. Electronegativity difference between bonding atoms should be very small or zero.
c. The elements should share the electrons mutually.
d. There should be 4 or more valance electrons.
e. The ionization energies of the elements must be high.
Example: HCl, Cl2, C6H6 and C2H2
Q.10 Why HCl has dipole-dipole forces of attraction?
Answer:
HCl forms a polar covalent bond atoms due to difference of electro negativity between
bonded atoms. There exists a dipole in the molecule. The positive end of one molecule attracts the negative end of their molecule. Hence dipole force. (Intermolecular forces) exist between HCl molecules.
Example:
![]()
Q.11 What is a triple covalent bond, explain with an example?
Answer:
When each bonded atom contributes three electrons, three bond pairs are involved in bond formation. This type of bond is called triple covalent bond.
Representation:
It is represented by (![]() ).
).
Example:
Triple covalent bond is formed in nitrogen molecule. In nitrogen molecule three bond pairs are involved in bond formation.
![]()
Q.12 What is difference between polar and non-polar covalent bonds, explain with one example of each?
Answer:
Difference between polar and non polar covalent
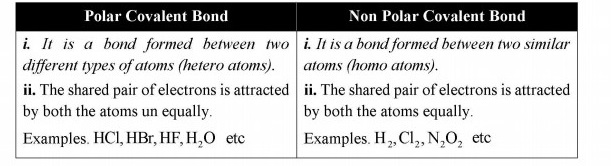
Q.13 Why a covalent bond becomes polar?
Answer:
When there is a difference of electronegativity between two covalently bonded atoms, there will be unequal attraction for the bond pair of electrons between such atoms. It will result in the formation of polar covalent bond.
Examples:
HCI, H2O etc.
Q.14 What is relationship between electronegativity and polarity?
Answer:
The polarity of a covalent bond depends upon the electronegativity difference between the bonded atoms. Higher the electronegativity difference between bonded atoms, greater will be thegolarity. Thus electronegativity and polarity are directly related:
Examples:
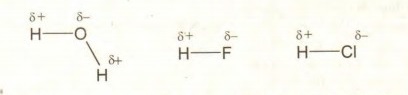
Q.15 Why does ice float on water?
Answer:
Ice floats on water because density of ice (0.917g/cm3) is less than that of liquid water
(1.00g/cm2) at 0°C.
Q.16 Give the characteristic properties of ionic compounds.
Answer:
Characteristics properties of ionic compounds.
i. Ionic compound are mostly crystalline solids.
ii. Ionic compounds are good conductors in solution and in molten form due to presence of free ions in them.
iii. Ionic compounds have high melting and boiling points. For example NaCl has melting point 800°C and boiling point 1413°C.
iv. Ionic compounds dissolve in polar solvents e.g. NaCl dissolves in water.
Q.17 What characteristic properties do the covalent compounds have?
Answer:
Characteristic properties of covalent compounds:
i. Melting boiling points: They have usually low melting and boiling point.
ii. Electrical conductivity: They are usually bad conductors of electricity. Polar compounds are conductors in their solutions in polar solvents.
iii. Solubility: They are usually insoluble in water but soluble in non-aqueous solvents like benzene, ether, alcohol and acetone.
iv. Crystal formation: Bigger molecules with three dimensional bonding form covalent crystals which are very stable and hard. They have high melting and boiling points.