Unit 2 – Structure of Atoms (Short Questions)
Q.1 What is the nature of charge on cathode rays?
Q.2 Give five characteristics of cathode rays.

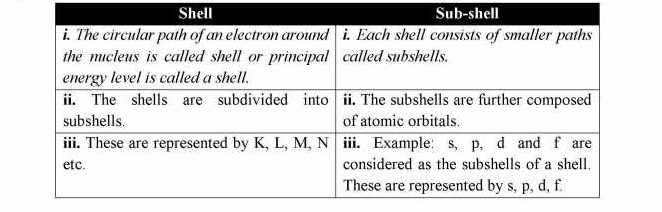
Q.4 Differentiate between shell and subshell with examples of each.
Q.5 An element has an atomic number 17. How many electrons are present in K, L and M shells of the atom?
Q.6 Write down the electronic configuration of Al3+. How many electrons are’ present in its outermost shell?
Q.7 Magnesium has electronic configuration 2, 8, 2,(a) How many electrons are in the outermost shell?(b) In which subshell of the outermost shell electrons are present?(c) Why magnesium tend to lose electrons?
Q.8 What will be the nature of charge on an atom when it loses an electron or when it gains an electron?
Q.9 For what purpose is U-235 used?
Q.10 A patient has goiter, how will it be detected?
Q.11 Give three properties of positive rays.
Q.12 What are the defects of Rutherford’s atomic model?
Q.13 As long as electron remains in an orbit, it does not emit or absorb energy. When does it emit or absorb energy?
Q.1 What is the nature of charge on cathode rays?
Answer:
Cathode rays are negatively charged particles. J.J. Thomson discovered the e/m (charge/mass) ratio of cathode rays and found it equal to electron.
Q.2 Give five characteristics of cathode rays.
Answer:
The characteristics of cathode rays are as under:
i. These rays travel in a straight line perpendicular to the cathode surface.
ii. They raise the temperature of the body on which they fall.
iii. Light is produced when these rays hit the sides of discharge tube.
iv. They can cast a sharp shadow of an opaque object if placed in their path.
v. The nature of rays does not depend upon the nature of as used in discharge tube.
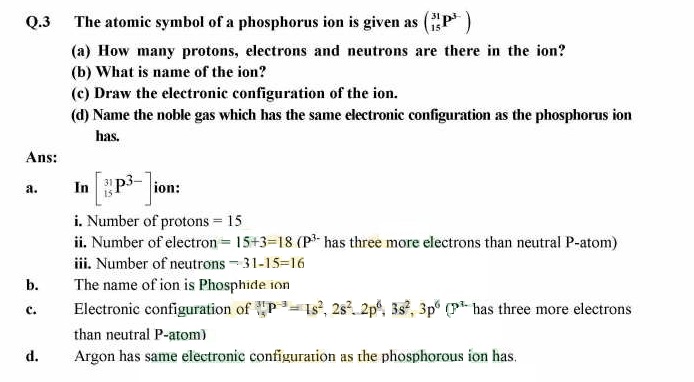
Q.4 Differentiate between shell and subshell with examples of each.
Answer:
Q.5 An element has an atomic number 17. How many electrons are present in K, L and M shells of the atom?
Answer:
Atomic number of element = number of electrons = 17 Therefore, its electronic configuration will be
K L M
2 8 7
OR
1s2, 2s2, sp6, 3s2, 3p5
Q.6 Write down the electronic configuration of Al3+. How many electrons are’ present in its outermost shell?
Answer:
Atomic number of Al = 13
Number of electrons of Al = 13
Number of electrons of A13 + = 13-3 = 10 electrons.
Thus electronic configuration of A13+ ion
K L
2 8
In terms of subshell: 1s2, 2s2, sp6
Therefore,
Number of electrons present in outer most shell of Al3+ = 8 electrons
Q.7 Magnesium has electronic configuration 2, 8, 2,
(a) How many electrons are in the outermost shell?
(b) In which subshell of the outermost shell electrons are present?
(c) Why magnesium tend to lose electrons?
Answer:
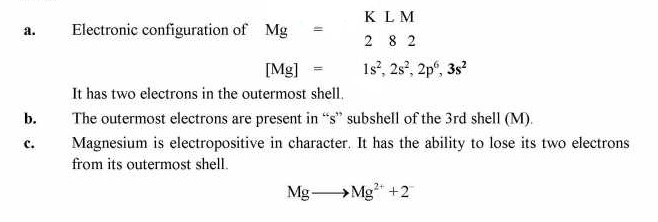
Q.8 What will be the nature of charge on an atom when it loses an electron or when it gains an electron?
Answer:
When an atom loses an electron, it acquires positive charge due to more number of protons in the nucleus e.g.
Na → Na1+ +le–
(2,8,1) (2,8)
When an atom gains an electron, it possesses negative charge due to more electrons than protons in the atom e.g.
Cl+le– → Cl-1
(2,8,7) (2,8,8)
Q.9 For what purpose is U-235 used?
Answer:
Radioactive isotope U-235 is used to generate electricity.
![]()
Q.10 A patient has goiter, how will it be detected?
Answer:
Isotopes of iodine-131 are used for diagnosis of goiter in thyroid gland. These radioactive isotopes are used as tracers in medicine to diagnose the presence of tumor in the human body.
Q.11 Give three properties of positive rays.
Answer:
Positive rays are also called “canal rays”. Their properties are:
i. These rays travel in straight line in a direction opposite to the cathode rays.
ii. These are positively charged rays.
iii. Mass of these particles was found equal to that of a proton or simple multiple of it.
Q.12 What are the defects of Rutherford’s atomic model?
Answer:
i. Stability of atom:
According to classical theory of radiations, electrons being charged particles should release or emit energy continuously. They should ultimately fall into the nucleus.
įi. Nature of spectrum:
If the electrons emit energy continuously they should form a continuous atomic spectrum but in fact, line atomic spectrum was observed.
Q.13 As long as electron remains in an orbit, it does not emit or absorb energy. When does it emit or absorb energy?
Answer:
Electrons do not emit or absorb energy till they remain in their orbits. Electron emits energy when it jumps from high energy level to the lower energy level. An electron absorbs energy when it jumps from a lower energy orbit to a higher energy orbit. The change in energy is given by the following Planck’s equation
AE = E2 – E = hv (Energy absorb)
Where
E1 = energy of lower energy orbit
E2 = energy of higher energy orbit
“h” is Planck’s” constant. Its value is 6.63×10-34 Js and frequency of light.
And
E2-E1=-hv (Energy emitted)
Where
Ei = energy of lower energy orbit
E2 = energy of higher energy orbit
“h” is Planck’s” constant. Its value is 6.63×10-34 Js and frequency of light.
And
E2-E1=-hv (Energy emitted)