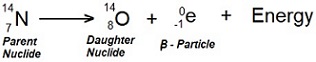Chapter 18 Atomic and Nuclear Physics (Long Questions)
18.1. What is difference between atomic number and atomic mass number? Give a symbolical representation of a nuclide.
18.2. What do you mean by the term radioactivity? Why some elements are radioactive but some are not?
18.3. How can we make radioactive elements artificially? Describe with a suitable example.
18.4. What are the three basic radioactive decay processes and how do they differ from each other?
18.5. Write the alpha decay process for . Identify the parent and daughter nuclei in ![]() this decay.
this decay.
18.6. Explain whether the atomic number can increase during nuclear decay. Support your answer with an example.
18.7. What do you understand by half-life of a radioactive element?
18.8. Is radioactivity a spontaneous process? Elaborate your answer with a simple experiment.
18.9. What is meant by background radiations? Enlist some sources of background radiations.
18.10. Describe two uses of radioisotopes in medicine, industry or research.
18.11. What are two common radiation hazards? Briefly describe the precautions that are taken against them.
18.12. Complete this nuclear reaction: ![]() . Does this reaction involve fission or fusion? Justify your answer.
. Does this reaction involve fission or fusion? Justify your answer.
18.13. Nuclear fusion reaction is more reliable and sustainable source of energy than nuclear fission chain reaction. Justify this statement with plausible arguments.
18.14. A nitrogen nuclide ![]() decays to become an oxygen nuclide by emitting an electron. Show this process with an equation.
decays to become an oxygen nuclide by emitting an electron. Show this process with an equation.
18.15. Determine which of these radioactive decay processes are possible:
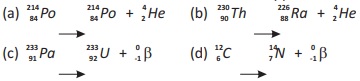
CONCEPTUAL QUESTIONS
18.1. Is it possible for an element to have different types of atoms? Explain.
18.2. What nuclear reaction would release more energy, the fission reaction or the fusion reaction? Explain.
18.3. Which has more penetrating power, an alpha particle or a gamma ray photon?
18.4. What is the difference between natural and artificial radioactivity?
18.5. How long would you likely have to wait to watch any sample of radioactive atoms completely decay?
18.6. Which type of natural radioactivity leaves the number of protons and the number of neutrons in the nucleus unchanged?
18.7. How much of a 1 g sample of pure radioactive substance would be left undecoyed after four half- lives?
18.8. Tritium, ![]() is radioactive isotope of hydrogen. It decays by emitting an electron. What is the daughter nucleus?
is radioactive isotope of hydrogen. It decays by emitting an electron. What is the daughter nucleus?
18.9. What information about the structure of the nitrogen atom can be obtained from its nuclide ![]() ? In what way atom in
? In what way atom in![]() is different from the atom in
is different from the atom in![]() ?
?
18.1. What is difference between atomic number and atomic mass number? Give a symbolical representation of a nuclide.
Ans. 1. Atomic Number (Z):
The number of protons in a nucleus is called its atomic number. It is denoted by Z.
2. Atomic Mass Number (A):
The number of nucleons in the nucleus is called Atomic Mass number. It is some of the masses of protons and neutrons in the nucleus. It is denoted by A.
Symbolic Representation:
Generally atom is represented by: X . It is called nuclide where X denotes element, Z is atomic number and A is, atomic mass number.
18.2. What do you mean by the term radioactivity? Why some elements are radioactive but some are not?
Ans. Radioactivity:
The spontantaneous emission, of radiation by unstable nuclei having atomic number greater than 82(Z> 82) is called natural radioactivity. The elements are called radioactive elements. The elements with atomic number (Z< 82) are stable, there is balance between short range attractive nuclear forces between nucleons and repulsive forces between : protons. But when the atomic number Z increases from 82 they become unstable and give out different radiations.
18.3. How can we make radioactive elements artificially? Describe with a suitable example.
Ans. Radioactive Isotopes OR Radioisotopes:
Artificially produced radioactive elements, by the bombardment of different particles, are called radioactive isotopes or radioisotopes. Elements having atomic number Z (I to 82) are stable and non radioactive elements can also be changed into unstable and radioactive elements by bombarding them with protons, neutrons or alpha particles.
Examples:
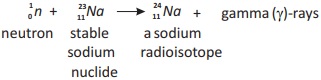
When sodium-l l is bombarded with neutrons, it becomes unstable and excited. Then it de-excites with the emission of y-ray photon as shown in nuclear equation.
18.4. What are the three basic radioactive decay processes and how do they differ from each other?
Ans. There are three basic radioactive decay processes which are represented by a nuclear equations.
For example, if unstable parent nuclide X changes into a daughter nuclide Y with the emission of alpha particle, beta particle or gamma particle.
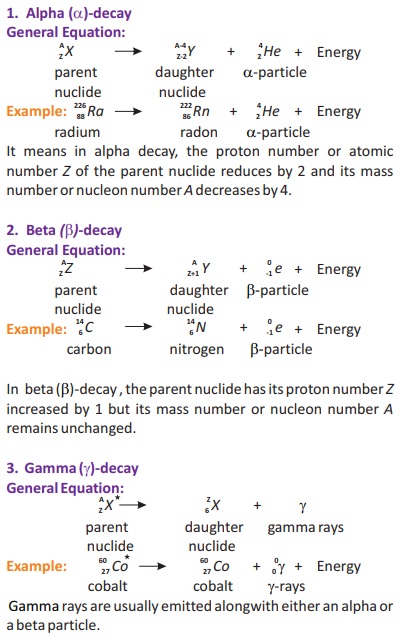
The nature of element does not change. It comes back to normal state after excited state.
Asterisk (*) sign: The sign (*) shows that nucleus is in excited state.
De-excitation: In de-excitation process extra energy is released in the form of γ-rays.
18.5. Write the alpha decay process for . Identify the parent and daughter nuclei in ![]() this decay.
this decay.
Ans. Following is the nuclear equation for (![]() )
)
![]()
18.6. Explain whether the atomic number can increase during nuclear decay. Support your answer with an example.
Ans. Yes, atomic number (Z) can increase during B-decay.
Example: Carbon -14 gives B-decay with Nitrogen-14. After B-decay its atomic number increases by 1 unit and mass number A remains same as shown in the nuclear equation.
18.7. What do you understand by half-life of a radioactive element?
Ans. Half Life: (T1/2)
The time during which half of the unstable nuclei disintegrate is called the half-life of the sample of radioactive element.
18.8. Is radioactivity a spontaneous process? Elaborate your answer with a simple experiment.
Ans. Yes, radioactivity is spontaneous and natural process. It does not depend upon any change of physical or chemical combination.
Experiment: A small quantity of the radium source was placed in a hole dug in block of lead.
The radiations emitted from radium are passed in the space between the two poles of permanent magnet placed inside an evacuated chamber. And photographic plate- is placed at right angle to the direction of these rays. When, after some time, the photographic plate was developed which show three separated spots. This proves the existence of three different types of radiations.
18.9. What is meant by background radiations? Enlist some sources of background radiations.
Ans. Background Radiations:
Radiations present in atmosphere due to different natural radioactive substances are called background radiations.
Sources:
1. In our environment the rocks, soil, water, and air are traces of radioactive elements.
2. Our Earth also receives radiations called cosmic radiations from outer space. These radiations are high-energy radiations, which consist of protons, electrons, alpha particles and larger nuclei.
3. The cosmic radiations interact with atoms in the atmosphere to create a shower of secondary radiations including X-rays, muons, protons, alpha particles, electrons, land neutrons.
18.10. Describe two uses of radioisotopes in medicine, industry or research.
Ans. Uses of Radio isotopes:
Radioisotopes are frequently used in medicine, industry and agriculture for variety of useful purposes.
Tracers:
1. Tracers are chemical compounds containing some quantity of radioisotope.
a. Tracers in Medical:
Tracers are used to explore the metabolism of chemical reactions inside the human body, animal or plant.
For example, isotopes of iodine -131 is accumulated in the thyroid gland and can be used for monitoring of thyroid functioning are used for diagnosis of goiter in thyroid gland.
b. Tracers in Industry:
Tracers are used to locate the wear and tear of the moving parts of the machinery. They can be used to locate the leaks in underground pipes.
c. Tracers in Agriculture:
The radioactive like Phophorous-32 is used as tracer to find out how well the plants are absorbing the phosphate fertilizer which are crucial to their growth.
2. Medical Treatment (Radiotherapy):
Radioisotopes are used ‘in nuclear medicine for curing various diseases. For example radioactive Cobalt-60is used for curing cancerous tumor and cells. The radiations kill the, cells of malignant tumor in the human body.
3. Carbon Dating: (Archeological and Geological uses):
It is the method, of age determination of old carbon containing objects (fossils) by measuring the radioactivity of C – 14 in them is called radio- carbon dating or simply carbon dating.
18.11. What are two common radiation hazards? Briefly describe the precautions that are taken against them.
Ans. Radiation is energy. It can come from unstable atoms that undergo radioactive decay, or it can be produced by machines.
There are two kinds of radiation:
1. Non-ionizing radiation: Non-ionizing radiation is described as a series of energy waves composed of oscillating electric and magnetic fields traveling at the speed of light. Non-ionizing radiation includes the spectrum of ultraviolet (UV), visible light, infrared (IR), microwave (MW), radio frequency (RF), and extremely low frequency (ELF).
2. Ionizing radiation: Ionizing radiation is a type of energy released by atoms that travels in the form of electromagnetic waves (gamma or X-rays) or particles (neutrons, beta or alpha). The spontaneous disintegration of atoms is called radioactivity, and the excess energy emitted is a form of ionizing radiation.
precautions:
You can work safely around radiation and/or contamination by following a few simple precautions:
1. Use time, distance, shielding and containment to reduce exposure.
2. Wear dosimeters (e.g., film or TLD badges) if issued.
3. Avoid contact with the contamination.
4. Wear protective clothing that, if contaminated, can be removed.
5. Wash with nonabrasive soap and water any part of the body that may have come in contact with the contamination.
6. Assume that all materials, equipment and personnel that came in contact with the contamination are contaminated. Radiological monitoring is recommended before leaving the scene.
18.12. Complete this nuclear reaction: ![]() . Does this reaction involve fission or fusion? Justify your answer.
. Does this reaction involve fission or fusion? Justify your answer.
Ans. As the heavy nuclei is broken into smaller nuclei, so it is a nuclear fission reaction.
![]()
18.13. Nuclear fusion reaction is more reliable and sustainable source of energy than nuclear fission chain reaction. Justify this statement with plausible arguments.
Ans. As the light nuclei are fused to give rise heavy nucleus in nuclear reaction. More energy is released in nuclear fusion as compared to fission reaction.
More than 200 Mev energy is released in one nuclear fusion reaction, Its main fuel is hydrogen that is easily available. The main hurdle in sustaining this reaction on earth is the temperature control because very high temperature is required to sustain this reaction.
18.14. A nitrogen nuclide ![]() decays to become an oxygen nuclide by emitting an electron. Show this process with an equation.
decays to become an oxygen nuclide by emitting an electron. Show this process with an equation.
Ans. Following a nuclear equation showing nitrogen nuclide (parent element) for β-decay.
18.15. Determine which of these radioactive decay processes are possible:
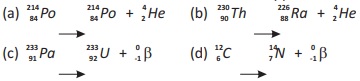
Ans. The above nuclear equations (b), (c) and (d) are possible.
CONCEPTUAL QUESTIONS
18.1. Is it possible for an element to have different types of atoms? Explain.
Ans. Yes, an element can have different atoms with same atomic number but different mass number, These are called isotopes of the element,
For example: There are three isotopes of hydrogen called Protium, Deuterium and Tritium.
18.2. What nuclear reaction would release more energy, the fission reaction or the fusion reaction? Explain.
Ans. The nuclear fusion reaction release more energy as compared to fission nuclear reaction.
Two hundred million electron volt (200Mev) energy is released in one reaction of fusion.
18.3. Which has more penetrating power, an alpha particle or a gamma ray photon?
Ans. Gamma ray photon has the highest penetrating power, it can pass through 2 cm thick of aluminum and can be blocked by lead.
18.4. What is the difference between natural and artificial radioactivity?
Ans. Natural Radioactivity: The spontaneous and natural decay of unstable elements (Z > 82) into another element with the emission of (α, β, or γ) radiations, is caked natural radioactivity.
Artificial Radioactivity: The process in which the stable and unstable nuclide can be changed into radioactive by the bombardment of particles like protons, neutrons etc.
18.5. How long would you likely have to wait to watch any sample of radioactive atoms completely decay?
Ans. It is difficult to measure the time until whole of the sample decay. It is supposed to be infinite time.
18.6. Which type of natural radioactivity leaves the number of protons and the number of neutrons in the nucleus unchanged?
Ans. Gamma (γ ) – decay:
General equation:
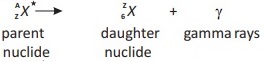
The nature of element does not change. It comes back to normal state after excited state.
Asterisk (*) sign: The sign (*) shows that nucleus is in excited state.
De-excitation: In de-excitation process extra energy is released in the form of y-rays.
18.7. How much of a 1g sample of pure radioactive substance would be left undecoyed after four half- lives?
Ans. Sample of pure radioactive matter = Ao = 1 gm
Matter left after first half- life = T 1/2 = 1/2 gm
Matter left after second half- life = 2 x T 1/2 = 1/4 gm
Matter left after third half- life = 3 x T 1/2 = 1/8 gm
Matter left after fourth half- life = 4 x T 1/2 = 1/16 gm
Hence only 0.0625gm of sample will be left after four half lives.
18.8. Tritium, ![]() is radioactive isotope of hydrogen. It decays by emitting an electron. What is the daughter nucleus?
is radioactive isotope of hydrogen. It decays by emitting an electron. What is the daughter nucleus?
Ans.
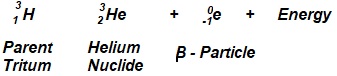
18.9. What information about the structure of the nitrogen atom can be obtained from its nuclide ![]() ? In what way atom in
? In what way atom in![]() is different from the atom in
is different from the atom in![]() ?
?
Ans. From the symbol ![]() it is clear Z = Atomic number, so number of protons = 7
it is clear Z = Atomic number, so number of protons = 7
Mass number A = sum of protons and neutrons = 14
so number of neutrons N = A – Z = 14 – 7 = 7
Hence it is one of the isotopes of Nitrogen. In Nitrogen atom there are 7 electrons.