Chapter 18 Numerical Problems Examples
Example 18.1: Find the number of protons and neutrons in the nuclide defined by . 13X6
Solution: From the symbol, we have Atomic number Z = number of protons =6
Atomic mass A = number of protons + number of neutrons = 13
But number of protons are 6, so number of neutrons will be 7.
So the element is an isotope of carbon-6, and is written as
13X6
NUCLEAR TRANSMUTATIONS
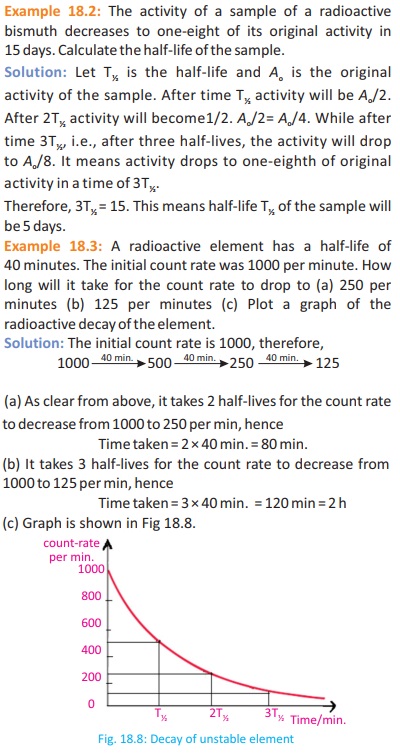
Example 18.4: The C-14: C-12 ratio in a fossil bone is found to be 1/4th that of the ratio in the bone of a living animal. The half- life of
C-14 is 5730 years what is the approximate age of the fossil?
Solution: Since the ratio has been reduced by factor of 4 therefore, two half-lives have passed.
Therefore age of the fossil is given by: 2 x 5730 = 11460 years
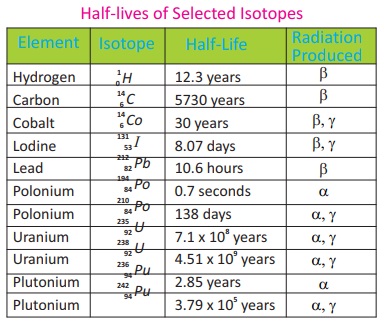
Half Lives of Selected Isotopes
<—————————–>