Unit 6 – Enzymes (Exercise Questions)
ANSWER THE FOLLOWING QUESTIONS.
Q.1. How would you define enzymes? Describe their characteristics.
Q.2. What do you mean by activation energy and why it is referred in the definition of enzymes?
Q.3. In a range of 0-35oC, the rate of reaction of an enzyme is proportional to temperature. Above 35oC and below 0oC, enzyme activity slows down and eventually stops. Explain why?
Q.4. How does pH affect enzymes activity?
Q.5. What characteristic of enzymes makes them specific for substrates?
Q.6. Briefly describe the factors that affect the activity of enzymes.
Q.7. Describe the lock and key mechanism of enzyme action.
SHORT QUESTIONS – TEXT EXERCISE
Q.1. Define cofactor and coenzyme.
Q.2. What is the main use of enzymes in paper industry?
THE TERMS TO KNOW
Q.1. How would you define enzymes? Describe their characteristics.
Answer:
Enzymes
Enzymes are proteins that catalyze i.e. speed up the biochemical reactions and are remained unchanged during the reaction. In enzyme catalyzed reactions, the molecules at the beginning of the process i.e. the substances on which the enzyme acts, are called substrates. The molecules which are produced at the end of the reaction are called products. Almost all the processes in a cell require enzymes for their occurrence at significant rates.
Enzymes posses the following important characteristics:
i. Almost all enzymes are proteins. which means they are made of amino acids ranging in number from 62 to over 2500. However some enzymes have no proteins such as some RNA molecules also act as biochemical catalysts.
ii. Enzymes are globular proteins.
iii. Like all proteins, enzymes are also made up of long and linear chains of amino acids, which fold and coil to produce a three- dimensional globular molecule.
iv. The reaction rates of most of the enzymes are millions of times faster than those of related uncatalyzed reactions. The enzymes are not consumed up at the end of the reaction like an other catalysts.
v. Enzymes are very specific for the type and nature of their substrates and reactions they catalyzed.
vi. The activities of enzymes are determined by’ a small portion of enzyme, molecule which is about 34.amino acids long and it is directly involved in the catalysis. This catalytic region of the enzyme is known as the active site. He active site recognized and binds the substrate and then carries out the biochemical reaction.
vii. As the enzymes are very specific for their reactions and substrates and speed up only related reactions, so the set of enzymes produced in a cell determines the type of metabolic pathways that occur in the cell.
viii. There are two categories of the enzymes on the basis of the site where they work i.e. inside or outside the cell. They may be extracellular enzymes such as pepsin which works outside the cells in the stomach cavity, or they may be intracellular enzymes such as enzymes of respiration and photosynthesis working inside the cells.
ix. The activity of the enzymes in the cells is regulated by a number of ways. The production of enzymes can be increased or decreased by the cells in response to the changes in the environment of the cell.
x. Some enzymes are complete catalytic units and do not need any additional compound to. work. While some other enzymes require non-protein molecules or metal ions for their activity. These non protein molecules are called co-factors. Cofactors are of three types.
a. It may be an inorganic ion or metal ion and is called activators.
b. It may be organic molecules and is tightly bound to the enzymes and are called prosthetic group e.g. heme.
c. It may be organic and loosely attacked to the enzyme and are called coenzymes. These are small organic molecules’ which transport chemical groups from one enzyme to the other.
Some of the important coenzyme are vitamins such as riboflavin, folic acid and thiamine.
xi. Many enzymes work together in a specific sequence and complete a particular pathway. For example in a metabolic pathway, one enzyme picks up I the products of another enzyme as a substrate and in turn passes on the products of its reaction to another enzymes for completion of the metabolic pathway.
Q.2. What do you mean by activation energy and why it is referred in the definition of enzymes?
Answer:
Enzymes
Enzymes are proteins that catalyze i.e. speed up the biochemical! reaction and are remained unchanged during the reaction. In enzyme catalyzed reactions, the molecules at the beginning of the process i.e. the substance!
on which the enzyme acts, are called substrates. The molecules which are produced at the end of the reaction are called products. Almost all the processes in a cell require enzymes for their occurrence at significant rates,
Enzymes work by lowering the requirement of activation energy. Actually, all the chemical reactions’ require activation energy to break the chemical bonds and start the reaction. This .need for activation energy acts as!
barrier for the, start of reactions and enzymes lower these barriers. So the reactions proceed at a faster rate in the presence of enzymes.
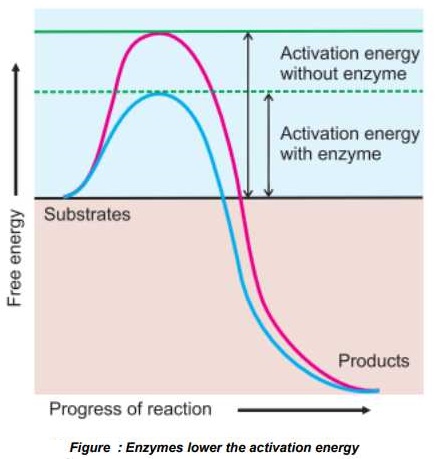
Enzymes lower the activation energy in a number of different ways, which include:
i. By altering the shape and structure of the substrates and decreasing the amount of energy required to complete the transaction.
ii. By disrupting the charge distribution on substrate molecules.
iii. By changing the orientation of the substrate to the right direction.
Q.3. In a range of 0-35oC, the rate of reaction of an enzyme is proportional to temperature. Above 35oC and below 0oC, enzyme activity slows down and eventually stops. Explain why?
Answer:
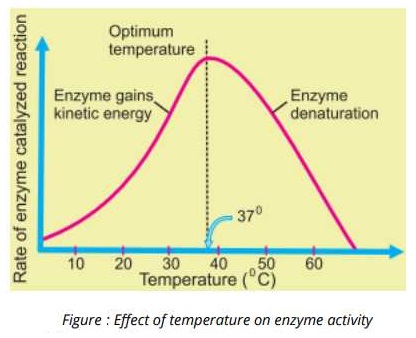
Temperature:
The rise in temperature speeds up the rate of an enzyme catalyzed reaction. Each enzymes works at its maximum rate of some specific temperature which is called the optimum temperature of that enzyme. When the temperature is increased is a certain value, the Heat provides activation energy and therefore, chemical reactions are accelerated at high temperatures. Heat also supplies kinetic energy to the reacting molecules, causing them to move rapidly. Thus the reactants move more quickly and chances of their collision with each other are increased. However, further increase in heat energy also increases the vibrations of atoms which make up the enzyme molecule. If the vibration become too violent, globular structure essential for enzyme activity is lost and the enzyme is said to be denatured, and the. condition is caned Denaturation, This results in the rapid. decline in the rat of enzyme action which may be stopped completely.
Q.4. How does pH affect enzymes activity?
Answer:
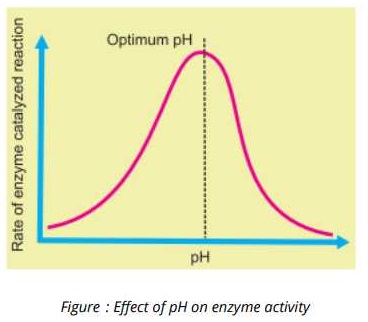
pH
Every enzyme functions most effective over a narrow range of pH known as the Optimum pH.
A slight change in pH above or below the optimum value can change ionization of the amino acids at the active site which results in retardation or complete blockage of enzyme activity.
Each enzyme has its specific value of optimum pH. For example pepsin present in the gastric juice of stomach, is active in acidic medium (low pH).
Similarly Trypsin which works in the small intestine, shows maxim activity in the alkaline medium (high pH).
Q.5. What characteristic of enzymes makes them specific for substrates?
Answer:
Specificity of Enzymes
More than 2r enzyme are known and each of these is specialized to carry out one specific reaction. Enzymes are also substrate specific.
The enzyme protease (which catalyze the break down of peptide bonds in proteins) will not work on any other molecules such as starch.
Similary, the enzyme lipase only acts on lipids and digests them in glycerol and fatty acids.
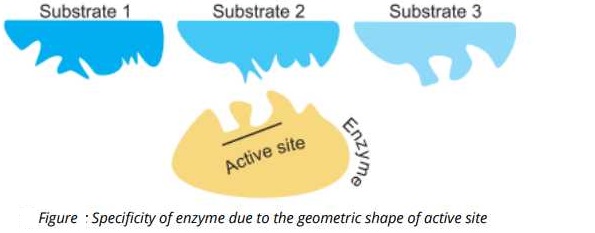
This specificity of different enzymes is determined by the shapes of their active sites. The active sites possess some specific geometric shapes that fit with the specific substrate only. Figure_____ shows, how the geometric
shape of the active site of enzymes fits into a substrate.
Practical Work
Exp. Perform an experiment to show the in vitro working of an enzyme. Enzyme can work both in-vivo and in-vitro. The in-vitro enzyme activity can be observed by an experiment. There are many examples, but we can select the protein digesting enzyme pepsin and the substrate meat to perform this experiment.
Problem: Can pepsin digest the proteins in meat?
Apparatus:
Meat, Pepsin solution, Test tubes, HCL, Biuret reagent.
Background Knowledge:
i. Animal meat contains lot of proteins.
ii. The enzyme pepsin is secreted in the stomach. It acts on proteins and digest them to peptides and amino acids.
Procedure:
1- Cut the meat in small pieces and add pieces of meat in two test tubes (one in each).
2- Then add lO-15mlof pepsin solution in one test tube and same amount of water in the other test tube which will act as control for comparison.
3- Then add 10 days of Bel in both the test tubes to create the acidic conditions.
4- Place both the test tubes in an incubator at 37°C for specific period of time.
Observation
1- Observe the pieces of meat after four hours.
2- Perform the Biuret test to confirm the presence or absence of proteins in the test tubes.
Results Of Biuret Test
The Biuret test gives negative results in the test tube in which pepsin and HCI were added. It shows that the proteins present in the test tube has been digested by he enzyme pepsin and hence proteins are absent.
Evaluation:
i- What effect did pH have on pepsin activity?
Ans: The acidic pH is requited for the activity of pepsin. It does not work in neutral or alkaline pH.
ii- What is the optimum pH of pepsin?
Ans: The optimum pH of pepsin is 1.5.
iii- An organism lives in a hot spring. What will be the effect on its enzymes if it is placed in cold water?
Ans: If an organism living in hot spring is placed in cold water its enzymes will not show any activity.
Experiment: Perform an experiment to demonstrate the in vitro working of amylase.
Practical Work
Perform an experiment to show the working of amylase in vitor Amylase is an enzyme that catalyses the breakdown of the polysaccharide. starch to the disaccharide maltose. It is present in saliva, plant tissues, and also, in seeds.
To observe the in-vitro enzyme activity we can also select starch .as substrate and amylase as the. starch digesting enzyme.
Apparatus required
Meat, Test tube, pepsin solution, Hcl, Biuret regent.
Background information.
• Starch turns iodine solution dark purple / black while disaccharides do not react with the iodine.
• Amylase enzyme is produced in oral cavity and pancreas. It acts on carbohydrate molecules and digests them to disaccharides (sugars made of two units)
Procedure
1. Prepare 1 % solution of amylase and put some of it in a test tube .
2. Add 2ml of starch solution in the test tube.
3. Incubate the test tube at 37°C for 15 minutes.
Observation
Observe the test tube after 15 minutes. Perform the iodine test to confirm the presence of starch. This can be done by putting few drops of iodine solution in the test tube. Observe the colour change in the test tube.
Results
The iodine test gives negative results. There was no color change. It confirms that no starch is present in the test tube and all have been digested into disaccharides.
Evaluation
(i) What ‘color appears when iodine test is positive?
(ii) Why was the experimental test tube incubated at 37°C?
(iii) If we perform .the iodine test on starch solution before putting it in amylase, what would be the results?
Q.6. Briefly describe the factors that affect the activity of enzymes.
Answer:
Factors affecting rate of enzyme action:
Enzymes are very sensitive biomolecules to the environment in which they work. Any change in the conditions / factors which alters the chemistry and shape of the enzyme, affects the activity of the enzyme. Some of these factors which affect the rate of enzyme action are described below:
1. Temperature:
The rise in temperature speeds up the rate of an enzyme catalyzed reaction .. Each enzymes works at its maximum rate of some specific temperature which is called the optimum temperature 01.\ that enzyme. When the temperature is increased is a certain value, the Heat provides activation energy and’ therefore, chemical reactions are
accelerated at high temperatures. Heat also supplies kinetic energy to the reacting molecules, causing them to move rapidly. Thus the reactants move more quickly and chances of their collision with each other are increased.
However, further increase in heat energy also increases the vibrations of atoms which make up the enzyme molecule. If the vibration become too violent, globular structure essential for enzyme activity is lost and the enzyme is said to be denatured, and the. condition is caned Denaturation, This results in the rapid. decline in the rat of enzyme action which may be stopped completely.

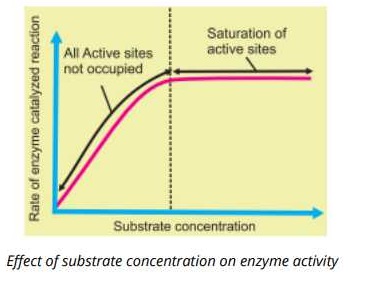
2. Substrate Concentration
If the active sites of the enzyme molecules are not fully filled, an increased in the substrate concentration results in increase of the reaction rate.
If the enzyme concentration is kept constant and the amount of substrate is increased, a point is reached when a further increase in the substrate d not increase the rate of the reaction any more. This is because high substrate level all the. active sites of the enzyme are occupied further increase in the substrate does not increase the reaction rate. This state is called saturation of the active sites and rate of reaction is not increased.
3. pH
Every enzyme functions most effective over a narrow range of pH known as the Optimum pH.
A slight change in pH above or below the optimum value can change ionization of the amino acids at the active site which results in retardation or complete blockage of enzyme activity.
Each enzyme has its specific value of optimum pH. For example pepsin present in the gastric-juice of stomach, is active in acidic medium (low pH). Similarly Trypsin which works in the small intestine, shows maxim activity in the alkaline medium (high pH).

Q.7. Describe the lock and key mechanism of enzyme action.
Answer:
Mechanism of Enzyme Action
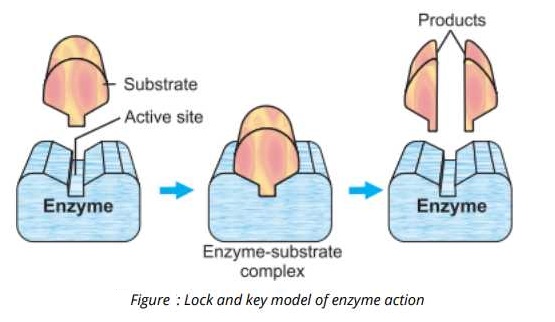
An enzyme and its substrate react with each other and form a temporary enzyme-substrate complex. The enzyme then brings about the catalysis of the reaction and the substrate is transformed into products. Finally the enzyme-substrate complex breaks and releases the enzyme and products.
Different mechanisms have been explained for enzyme reaction. Some of these include:
1- Lock and Key Model:
A German chemist Emil Fishers in 8Q4 proposed the lock and key model to explain the mechanism of enzyme action.
According to this model, both the enzyme and the substrate have specific complementary geometric shapes that fit exactly into each other like a lock and its key. This model also explains enzyme specificity.
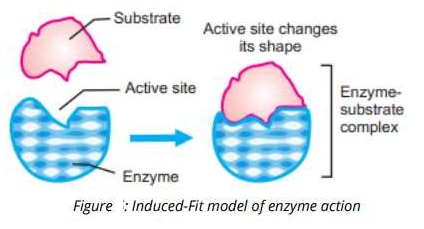
2- Induced Fit Model:
An American biologist Daniel Koshland in 1958, proposed a modification to the Fisher’s lock and key model and called it “induced-fit model”. The induced-fit model is more real and acceptable as compared to lock and key model. He explained that the enzymes are flexible structures and their active sites are reshaped as the substrates attack to the enzymes. This also explained that the active site is not a rigid structure rather it is moulded into
a precise portion to fit the substrate and perform its function.
SHORT QUESTIONS – TEXT EXERCISE
Q.1. Define cofactor and coenzyme.
Answer:
Some enzymes do not need any additional component to work. However, other require non-protein molecules or ions called cofactors. Cofactors can be either inorganic (e.g. metal ions) or organic (e.g. flavin and heme), If organic cofactors are loosely attached with enzyme they are called coenzymes. Coenzymes transport chemical groups from one enzyme to another. Some important vitamins (e.g. riboflavin, thiamine and folic acid) act as coenzymes.
Q.2. What is the main use of enzymes in paper industry?
Answer:
Paper Industry:
Enzymes degrade starch which results in the lowering its viscosity that helps in making paper.
THE TERMS TO KNOW